



Point-of-Care Diagnostics
•
CRISPR Engineering
•
Next-gen Imaging
•
Spatial Multiomics
•
Synthetic Biology
•
Biomaterial Design
Point-of-Care Diagnostics • CRISPR Engineering • Next-gen Imaging • Spatial Multiomics • Synthetic Biology • Biomaterial Design

Our Mission
Integrating engineering perspectives and disease biology to improve healthcare with precision, adaptability, and portability.
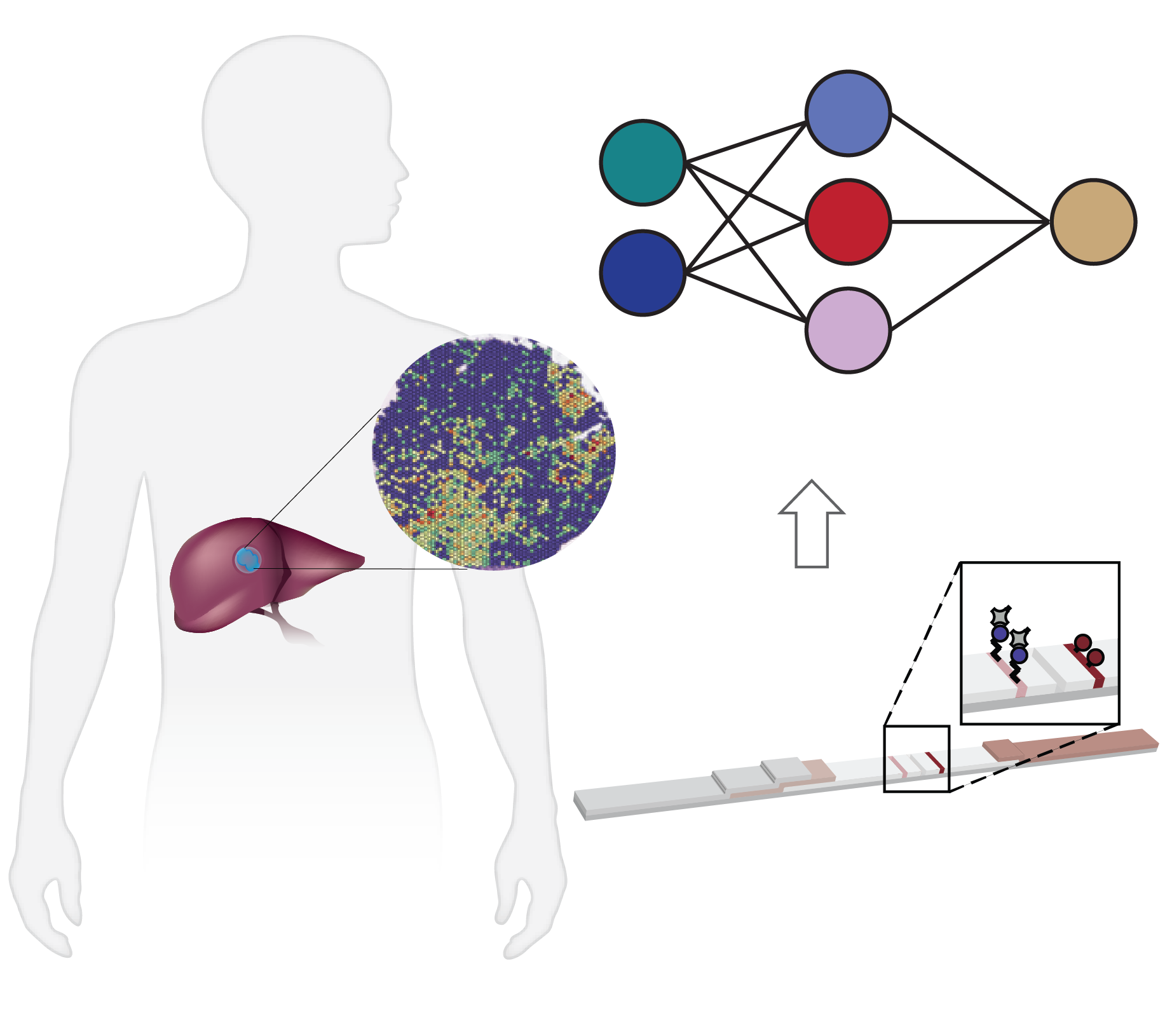
Virtually every biological process in development, physiology, and pathogenesis, involves dynamic changes in the abundance and activities of proteins. Our group develops enabling biotechnologies to study and modify spatiotemporal protein activities in complex biological systems including cells, tissues and whole organisms. Positioned at the living interface of precision engineering and health, we harness these new biological insights to tackle grand challenges in precision medicine, including noninvasive detection, timely interventions, and access in low-resource areas. Specifically, we will integrate chemical and synthetic biology, biomolecular and cellular engineering, spatial-omics, and systemic imaging to advance the understanding of holistic spatial-function relationships in health and disease, and ultimately realize the untapped potential of activity-based diagnostics and therapeutics. We are currently advancing three interconnected research directions: (1) spatial mapping of tissue activity, (2) activity-based diagnostics, and (3) conditionally activated therapeutics. We seek to use these insights to implement precision medicine in healthcare practice.
-
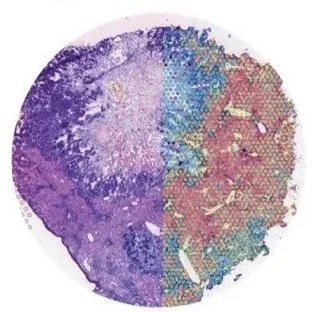
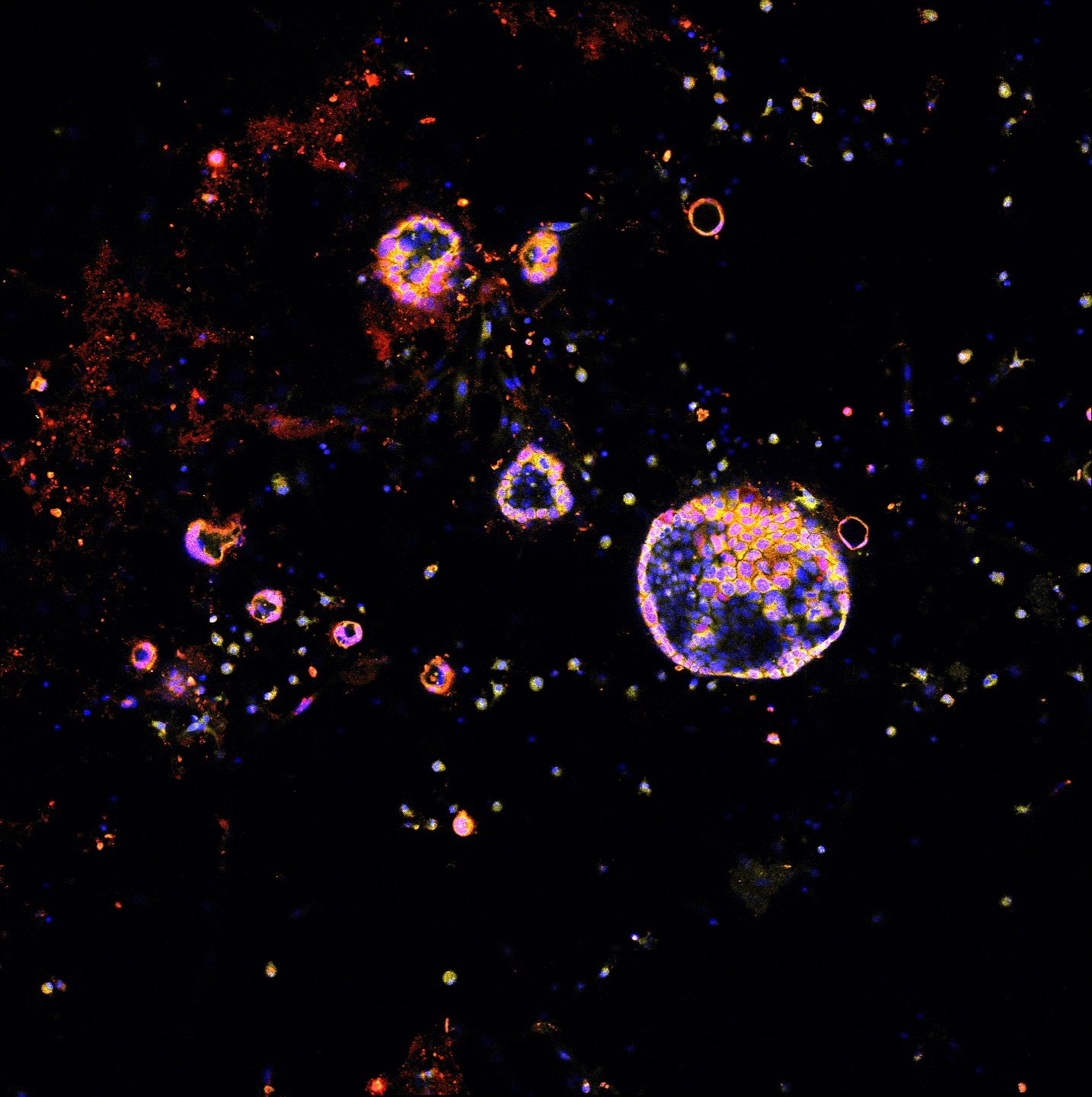
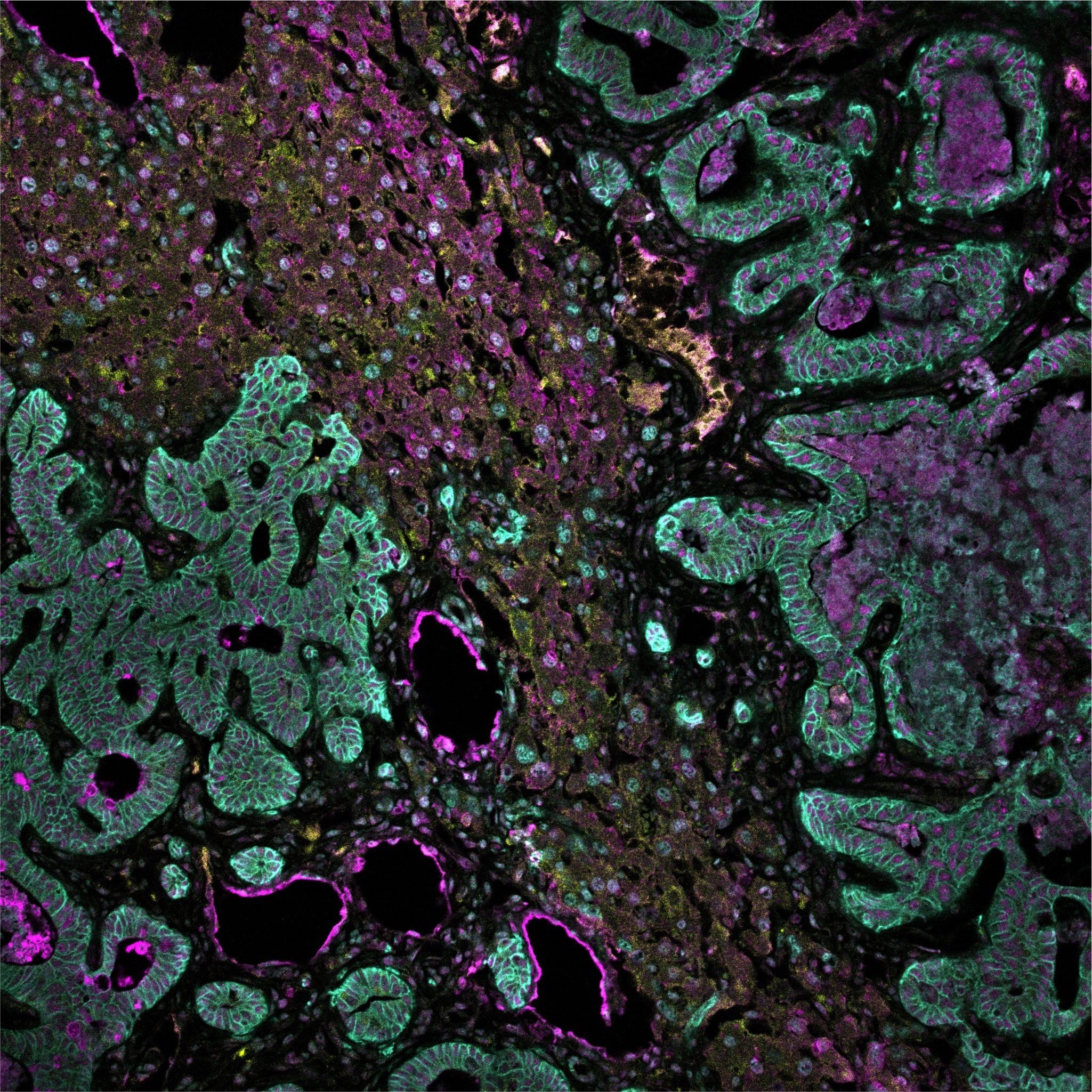
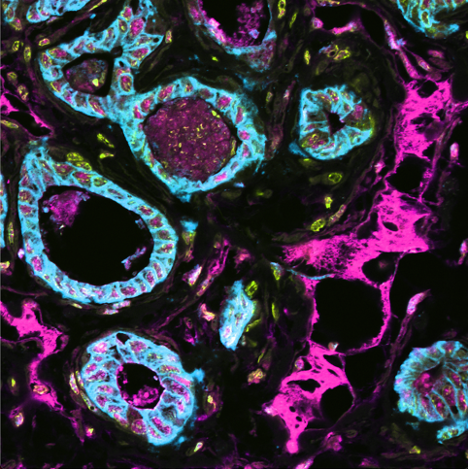
Spatial multi-omics has been revolutionary, allowing us to measure the abundance of various biomolecules (RNAs, proteins, metabolites), but not their activity levels, a distinct axis of regulation closely correlated to actuated biological function. Leveraging multiscale activity-based technologies, we are uniquely equipped to profile spatial activity within the context of intact organisms, which is critical to expanding our understanding of biological function and accelerating the development of the following next-gen precision diagnostics and therapeutics.
-
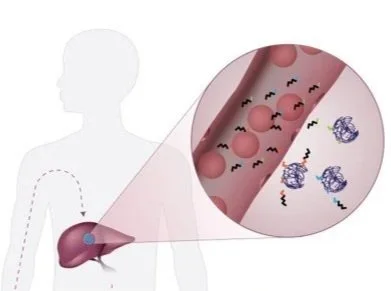
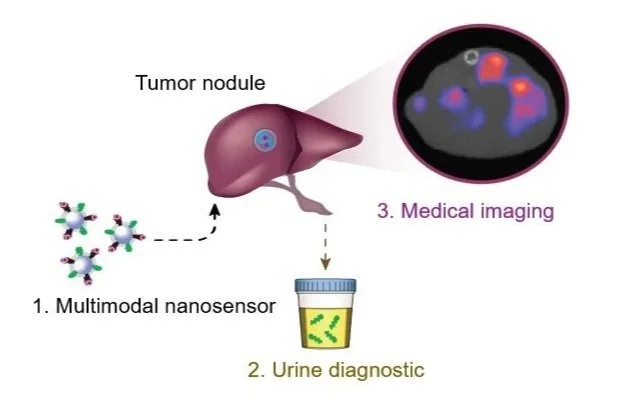
Enzyme activities provide signal amplification and the opportunity for a highly sensitive diagnostic. We develop smart diagnostics harnessing such biochemistry to measure or produce biomarkers of disease. These activity-based diagnostics can be engineered ranging from molecular probes to engineered cells and can be detected with diverse readout modalities for cancer, inflammation, and beyond. We will attempt to develop point-of-care diagnostics to assess disease signatures for next-gen liquid biopsy.
-
Non-viral delivery of biologics to extrahepatic cells remains a major challenge in therapeutics. To overcome such obstacles, we establish strategies to evolve and stringently select: delivery vehicles enabling specific delivery to desired tissues upon disease-associated enzyme activation, and functional precision of cargos with therapeutic potency. Particularly, we therapeutically program RNAs and functionally annotate noncoding RNAs as biomarkers or therapeutic targets.